What were the goals of this study?
People with MS (PwMS) switch DMTs for a variety of reasons, such as disease activity, side effects, insurance coverage, or other factors. Having accurate and complete information about available DMT choices, and support throughout the process, can help reduce the stress associated with switching and ensure a successful transition to the new DMT.
The main goal of this study was to survey PwMS about their experiences with switching and starting DMTs, and understand their needs for information and support during this process. Other goals included measuring familiarity with specific DMT-related terms, and learning about the use of digital apps for managing MS.
Who led the study and what was the funding source?
The study was led by a team from Sandoz (Shelby Marchese, Anna Chen, and Deedar Singh) and iConquerMS (Hollie Schmidt and Sara Loud). Sandoz provided the funding for the study.
How was the study conducted?
The study team developed a survey exploring the experience of switching DMTs and the use of patient support programs offered by DMT manufacturers. Questions were also included about awareness of the terms “biosimilar” and “biologic” and the use of digital apps to monitor and manage different aspects of MS.
iConquerMS members were invited to participate, with special outreach efforts made to natalizumab users. The survey was made available during April and May 2023.
What did we learn from this study?
315 PwMS initiated the survey, and 303 PwMS completed it.
- Most participants (86%) were currently using a DMT, and 71% had switched DMTs at some point.
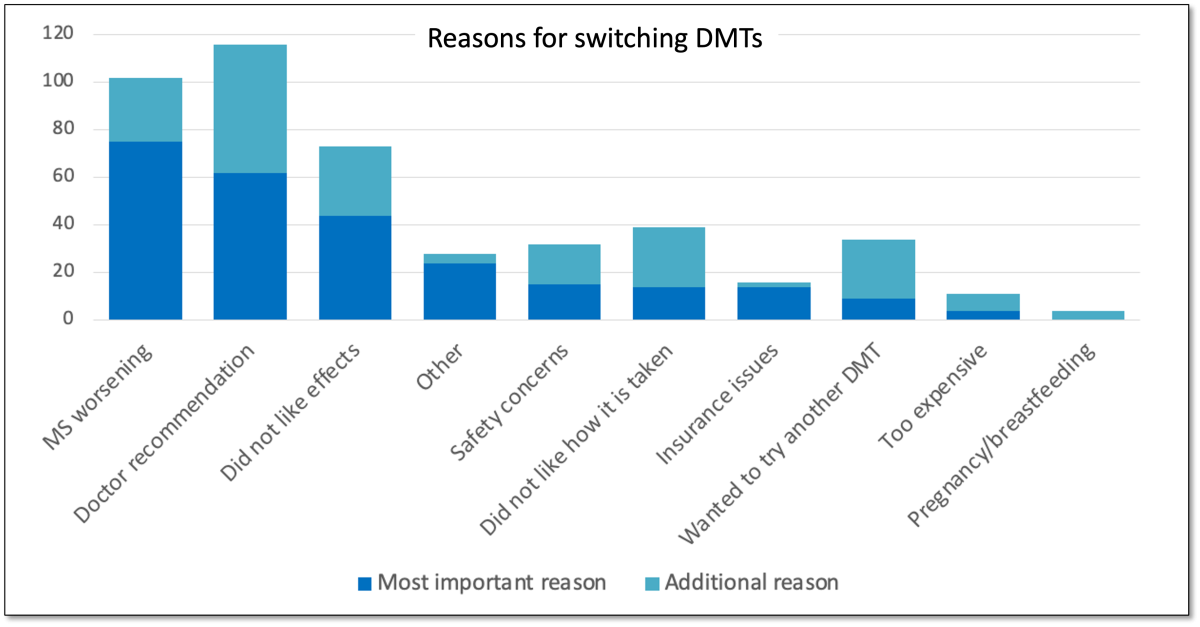
- Worsening MS, doctor recommendation, and side effects were the most common reasons cited for deciding to switch DMTs.
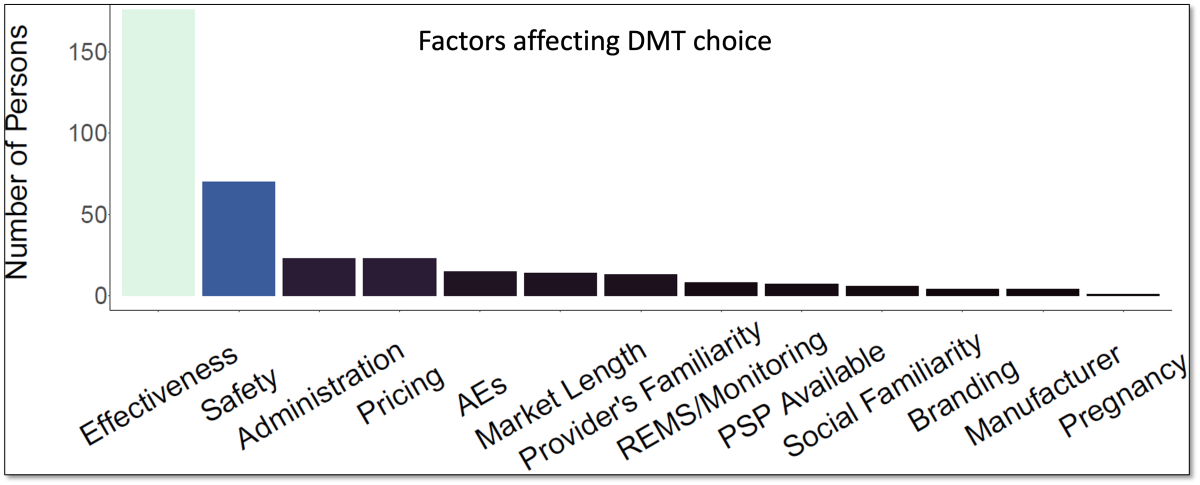
- When asked about the most important factor influencing their choice of DMT when switching, 69% of the participants selected DMT effectiveness. Safety, route of administration, and out-of-pocket costs were the next most commonly chosen factors.
- 82% had used a patient support program offered by a DMT manufacturer. Participants ranked payment assistance as the most helpful type of program.
- Just over half (51%) of participants had heard the term "biologic," and 43% had heard the term "biosimilar." However, not everyone who had heard these terms were able to define them.
- Only 26% of respondents had ever used digital apps or tools for monitoring their MS. Lack of interest and awareness were the most common reasons for not using these products.
What do these study findings mean?
These findings help to shed light on the decision-making process regarding DMT switches, and the types of information and support that PwMS need to successfully manage this process. We found that manufacturer support programs are viewed as helpful, and that explanations of potentially unfamiliar terms may be needed as new types of DMTs become available.
What will we do next with this information?
These results have been shared as a poster presentation at the 2024 Consortium of MS Centers annual meeting.
Learn more
Poster: Results shared at the 2024 CMSC annual meeting
Share this project summary with others:
https://www.iconquerms.org/experiences-ms-treatments-and-support-programs